Posted by glbgn754
on January 31, 2012
Uncategorized /
No Comments

Pill imprint AN 627 has been identified as Tramadol hydrochloride 50 mg.
Tramadol is used in the treatment of pain; back pain; fibromyalgia; rheumatoid arthritis; restless legs syndrome (and more), and belongs to the drug class miscellaneous analgesics. Risk cannot be ruled out during pregnancy.
Tramadol 50 mg is not subject to the Controlled Substances Act.
Tramadol hydrochloride
- Imprint(s):
- AN 627
|
- Strength(s):
- 50 mg
|
- Color:
- White
|
- Size:
- 9.00 mm
|
- Shape:
- Round
|
- Availability:
- Prescription only
|
- Drug Class:
- Miscellaneous analgesics
- Pregnancy Category:
- C – Risk cannot be ruled out
- CSA Schedule:
- Other Manufacturers / Repackagers:
-
| NDC Code |
Manufacturer / Repackager |
| 10135-0519 |
Marlex Pharmaceuticals Inc |
| 00904-6119 |
Major Pharmaceuticals |
| 54569-5967 |
A S Medication Solutions LLC (repackager) |
| 53002-0432 |
Pharmedix (repackager) |
| 54868-4638 |
Physicians Total Care Inc (repackager) |
| 66336-0915 |
Dispensing Solutions Inc. (repackager) |
| 33261-0105 |
Aidarex Pharmacuticals LLC (repackager) |
| 43063-0055 |
PDRX Pharmaceuticals Inc (repackager) |
| 18837-0157 |
Innoviant Pharmacy Inc (repackager) |
| 68387-0900 |
Keltman Pharmaceuticals Inc (repackager) |
| 10544-0327 |
Blenheim Pharmacal Inc (repackager) |
| 55289-0719 |
PDRX Pharmaceuticals Inc (repackager) |
Posted by glbgn754
on January 25, 2012
Uncategorized /
No Comments
APO CI 10
Pill imprint APO CI 10 has been identified as Citalopram hydrobromide 10 mg.
Citalopram is used in the treatment of depression; anxiety and stress; postpartum depression; severe mood dysregulation; obsessive compulsive disorder (and more), and belongs to the drug class selective serotonin reuptake inhibitors. Risk cannot be ruled out during pregnancy.
Citalopram 10 mg is not subject to the Controlled Substances Act.
See also related documents.
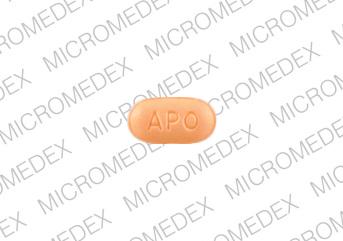

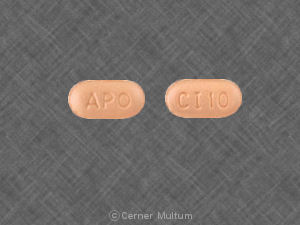
Citalopram hydrobromide
- Imprint(s):
- APO
CI 10
|
- Strength(s):
- 10 mg
|
- Color:
- Beige
|
- Size:
- 7.00 mm
|
- Shape:
- Elliptical / Oval
|
- Availability:
- Prescription only
|
- Inactive Ingredients:
-
- croscarmellose sodium
- lactose monohydrate
- magnesium stearate
- microcrystalline cellulose
- hydroxyethyl cellulose
- polyethylene glycol
- ferric oxide red
- titanium dioxide
- ferric oxide yellow
- Drug Class:
- Selective serotonin reuptake inhibitors
- Pregnancy Category:
- C – Risk cannot be ruled out
- CSA Schedule:
- N – Not a controlled drug
- Manufacturer:
- Apotex Corp.
- National Drug Code (NDC):
- 60505-2518
Posted by glbgn754
on January 25, 2012
Uncategorized /
No Comments
PALI 6
Generic Name: paliperidone
Pill imprint PALI 6 has been identified as Invega 6 mg.
Invega is used in the treatment of depression; schizophrenia; schizoaffective disorder; bipolar disorder and belongs to the drug class atypical antipsychotics. Risk cannot be ruled out during pregnancy.

Invega
- Generic Name:
- paliperidone
- Imprint(s):
- PALI 6
|
- Strength(s):
- 6 mg
|
- Color:
- Beige
|
- Size:
- 11.00 mm
|
- Shape:
- Elliptical / Oval
|
- Availability:
- Prescription only
|
- Inactive Ingredients:
-
- carnauba wax
- cellulose acetate
- polyethylene glycol
- propylene glycol
- povidone
- sodium chloride
- stearic acid
- butylated hydroxytoluene
- hypromelloses
- titanium dioxide
- ferrosoferric oxide
- Drug Class:
- Atypical antipsychotics
- Pregnancy Category:
- C – Risk cannot be ruled out
- CSA Schedule:
- N – Not a controlled drug
- Manufacturer:
- Janssen Pharmaceuticals
Posted by glbgn754
on January 25, 2012
Uncategorized /
No Comments
Pill imprint M 424 has been identified as Hydrochlorothiazide and metoprolol 25 mg / 50 mg.
Hydrochlorothiazide/metoprolol is used in the treatment of high blood pressure and belongs to the drug class antihypertensive combinations. Risk cannot be ruled out during pregnancy.
Hydrochlorothiazide/metoprolol 25 mg / 50 mg is not subject to the Controlled Substances Act.

Hydrochlorothiazide and metoprolol
- Imprint(s):
- M 424
|
- Strength(s):
- 25 mg / 50 mg
|
- Color:
- Beige
|
- Size:
- 10.00 mm
|
- Shape:
- Round
|
- Availability:
- Prescription only
|
- Inactive Ingredients:
-
- lactose anhydrous
- silicon dioxide
- croscarmellose sodium
- FD&C Yellow No. 6
- magnesium stearate
- microcrystalline cellulose
- povidone
- corn starch
- sodium lauryl sulfate
- Drug Class:
- Antihypertensive combinations
- Pregnancy Category:
- C – Risk cannot be ruled out
- CSA Schedule:
- N – Not a controlled drug
- Manufacturer:
- Mylan Pharmaceuticals Inc.
Posted by glbgn754
on January 25, 2012
Uncategorized /
No Comments
25 43 V
Pill imprint 25 43 V has been identified as Clonidine hydrochloride 0.3 mg.
Clonidine is used in the treatment of pain; adhd; high blood pressure; anxiety; opiate withdrawal (and more), and belongs to the drug class antiadrenergic agents, centrally acting. Risk cannot be ruled out during pregnancy.
Clonidine 0.3 mg is not subject to the Controlled Substances Act.
See also related documents.

Clonidine hydrochloride
- Imprint(s):
- 25 43
V
|
- Strength(s):
- 0.3 mg
|
- Color:
- Beige
|
- Size:
- 8.00 mm
|
- Shape:
- Elliptical / Oval
|
- Availability:
- Prescription only
|
- Inactive Ingredients:
-
- calcium phosphate dibasic anhydrous
- FD&C Yellow No. 6
- lactose monohydrate
- magnesium stearate
- microcrystalline cellulose
- sodium lauryl sulfate
- sodium starch glycolate type A potato
Posted by glbgn754
on January 25, 2012
Uncategorized /
No Comments
WW 120
Pill imprint WW 120 has been identified as Ergotamine tartrate and caffeine 1 mg / 100 mg.
Caffeine/ergotamine is used in the treatment of migraine; cluster headaches and belongs to the drug class antimigraine agents. Not for use in pregnancy.
Caffeine/ergotamine 1 mg / 100 mg is not subject to the Controlled Substances Act.
See also related documents.

Ergotamine tartrate and caffeine
- Imprint(s):
- WW 120
|
- Strength(s):
- 1 mg / 100 mg
|
- Color:
- Beige
|
- Size:
- 10.00 mm
|
- Shape:
- Round
|
- Availability:
- Prescription only
|
- Inactive Ingredients:
-
- corn starch
- sugar 6x powder
- mannitol
- compressible sugar
- microcrystalline cellulose
- silicon dioxide colloidal
- sodium starch glycolate
- magnesium
- FD&C Blue No. 2 Lake
- FD&C Yellow No. 6 Lake
- methylcellulose
- amide resin
- black pigment
- natural resin
- wax
Posted by glbgn754
on January 25, 2012
Uncategorized /
No Comments
LOGO TH
Generic Name: thyroid desiccated
Pill imprint LOGO TH has been identified as Armour thyroid 240 mg.
Armour Thyroid is used in the treatment of underactive thyroid; hypothyroidism, after thyroid removal; hashimoto’s disease; thyroid cancer; tsh suppression and belongs to the drug class thyroid drugs. Studies show no risk during pregnancy.
Armour Thyroid 240 mg is not subject to the Controlled Substances Act.

Armour thyroid
- Generic Name:
- thyroid desiccated
- Imprint(s):
- LOGO TH
|
- Strength(s):
- 240 mg
|
- Color:
- Beige
|
- Size:
- 11.00 mm
|
- Shape:
- Round
|
- Availability:
- Prescription only
|
Posted by glbgn754
on January 18, 2012
Uncategorized /
No Comments
B 293
Drug: Oxcarbazepine
Strength(s): 300 mg
Imprint: B 293
Color: Beige
Shape: Elliptical / Oval
Pill imprint B 293 has been identified as Oxcarbazepine 300 mg.
Oxcarbazepine is used in the treatment of bipolar disorder; seizures; anxiety; trigeminal neuralgia and belongs to the drug class dibenzazepine anticonvulsants. Risk cannot be ruled out during pregnancy.
Oxcarbazepine 300 mg is not subject to the Controlled Substances Act.
See also related documents.
Posted by glbgn754
on January 18, 2012
Uncategorized /
No Comments
Drug: Wellbutrin XL
Strength(s): 150 mg
Imprint: WELLBUTRIN XL 150
Color: Beige
Shape: Round
Generic Name: bupropion
Pill imprint WELLBUTRIN XL 150 has been identified as Wellbutrin XL 150 mg.
Wellbutrin XL is used in the treatment of depression; seasonal affective disorder and belongs to the drug class miscellaneous antidepressants. Risk cannot be ruled out during pregnancy.
Wellbutrin XL 150 mg is not subject to the Controlled Substances Act.
See also related documents.
- Generic Name:
- bupropion
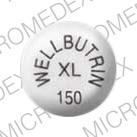
| Imprint(s):WELLBUTRIN XL 150 |
Strength(s):150 mg |
| Color:Beige |
Size:N/A |
| Shape:Round |
Availability:Prescription only |
- Drug Class:
- Miscellaneous antidepressants
- Pregnancy Category:
- C – Risk cannot be ruled out
- CSA Schedule:
- N – Not a controlled drug
- Manufacturer:
- GlaxoSmithKline
- National Drug Code (NDC):
- 00173-0730
Posted by glbgn754
on January 18, 2012
Uncategorized /
No Comments
Drug: Oxycodone Hydrochloride Extended-Release
Strength(s): 80 mg
Imprint: G 164
Color: Beige
Shape: Round
Pill imprint G 164 has been identified as Oxycodone hydrochloride extended-release 80 mg.
Oxycodone is used in the treatment of pain and belongs to the drug class narcotic analgesics. FDA has not classified the drug for risk during pregnancy.
Oxycodone 80 mg has a high potential for abuse. The drug has a currently accepted medical use in treatment in the United States or a currently accepted medical use with severe restrictions. Abuse of the drug may lead to severe psychological or physical dependence.
See also related documents.












