Posted by glbgn754
on January 31, 2012
Uncategorized /
No Comments
Clonazepam is used in the treatment of anxiety; bipolar disorder; panic disorder; seizure prevention; periodic limb movement disorder (and more), and belongs to the drug classes benzodiazepine anticonvulsants, benzodiazepines. There is positive evidence of human fetal risk during pregnancy.
Clonazepam 2 mg has a low potential for abuse. The drug has a currently accepted medical use in treatment in the United States. Abuse of the drug may lead to limited physical dependence or psychological dependence relative to the drugs in schedule 3.
Clonazepam
- Imprint(s):
- 93 834
|
- Strength(s):
- 2 mg
|
- Color:
- White
|
- Size:
- 8.00 mm
|
- Shape:
- Round
|
- Availability:
- Prescription only
|
- Inactive Ingredients:
-
- lactose monohydrate
- magnesium stearate
- microcrystalline cellulose
- povidone
- water
- corn starch
- Drug Class:
- Benzodiazepine anticonvulsants
Benzodiazepines
- Pregnancy Category:
- D – Positive evidence of risk
- CSA Schedule:
- 4 – Some potential for abuse
- Manufacturer:
- Teva Pharmaceuticals USA
- National Drug Code (NDC):
- 00093-0834

Posted by glbgn754
on January 31, 2012
Uncategorized /
No Comments
Generic Name: acetaminophen/oxycodone
Pill imprint 54 543 has been identified as Roxicet 325 mg / 5 mg.
Roxicet is used in the treatment of pain and belongs to the drug class narcotic analgesic combinations. Risk cannot be ruled out during pregnancy.
Roxicet 325 mg / 5 mg has a high potential for abuse. The drug has a currently accepted medical use in treatment in the United States or a currently accepted medical use with severe restrictions. Abuse of the drug may lead to severe psychological or physical dependence.
- Generic Name:
- acetaminophen/oxycodone
- Imprint(s):
- 54 543
|
- Strength(s):
- 325 mg / 5 mg
|
- Color:
- White
|
- Size:
- 11.00 mm
|
- Shape:
- Round
|
- Availability:
- Prescription only
|
- Inactive Ingredients:
-
- Drug Class:
- Narcotic analgesic combinations
- Pregnancy Category:
- C – Risk cannot be ruled out
- CSA Schedule:
- 2 – High potential for abuse
- Manufacturer:
- Roxane Laboratories, Inc.
- National Drug Code (NDC):
- 00054-4650
- Other Manufacturers / Repackagers
Posted by glbgn754
on January 31, 2012
Uncategorized /
No Comments
Generic Name: acetaminophen/oxycodone
Pill imprint Endo 602 has been identified as Endocet 325 mg / 5 mg.
Endocet is used in the treatment of pain and belongs to the drug class narcotic analgesic combinations. Risk cannot be ruled out during pregnancy.
Endocet 325 mg / 5 mg has a high potential for abuse. The drug has a currently accepted medical use in treatment in the United States or a currently accepted medical use with severe restrictions. Abuse of the drug may lead to severe psychological or physical dependence.
Endocet
- Generic Name:
- acetaminophen/oxycodone
- Imprint(s):
- Endo 602
|
- Strength(s):
- 325 mg / 5 mg
|
- Color:
- White
|
- Size:
- 15.00 mm
|
- Shape:
- Round
|
- Availability:
- Prescription only
|
- Inactive Ingredients:
-
- silicon dioxide
- croscarmellose sodium
- crospovidone
- microcrystalline cellulose
- povidone
- corn starch
- stearic acid
- Drug Class:
- Narcotic analgesic combinations
- Pregnancy Category:
- C – Risk cannot be ruled out
- CSA Schedule:
- 2 – High potential for abuse
- Manufacturer:
- Endo Pharmaceuticals Inc.

Posted by glbgn754
on January 31, 2012
Uncategorized /
No Comments
Pill imprint MYLAN A4 has been identified as Alprazolam 2 mg.
Alprazolam is used in the treatment of anxiety; depression; panic disorder; tinnitus; dysautonomia and belongs to the drug class benzodiazepines. There is positive evidence of human fetal risk during pregnancy.
Alprazolam 2 mg has a low potential for abuse. The drug has a currently accepted medical use in treatment in the United States. Abuse of the drug may lead to limited physical dependence or psychological dependence relative to the drugs in schedule 3.
Alprazolam
- Imprint(s):
- MYLAN A4
|
- Strength(s):
- 2 mg
|
- Color:
- White
|
- Size:
- 10.00 mm
|
- Shape:
- Round
|
- Availability:
- Prescription only
|
- Inactive Ingredients:
-
- silicon dioxide
- croscarmellose sodium
- docusate sodium
- lactose monohydrate
- magnesium stearate
- microcrystalline cellulose
- sodium benzoate
- sodium lauryl sulfate
- Drug Class:
- Benzodiazepines
- Pregnancy Category:
- D – Positive evidence of risk
- CSA Schedule:
- 4 – Some potential for abuse
- Manufacturer:
- Mylan Pharmaceuticals Inc.
- National Drug Code (NDC):
- Pill imprint M 4 has been identified as Hydromorphone hydrochloride 4 mg.
-
Posted by glbgn754
on January 31, 2012
Uncategorized /
No Comments
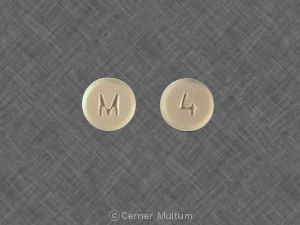
Pill imprint M 4 has been identified as Hydromorphone hydrochloride 4 mg.
Hydromorphone is used in the treatment of pain; cough; anesthetic adjunct and belongs to the drug class narcotic analgesics. Risk cannot be ruled out during pregnancy.
Hydromorphone 4 mg has a high potential for abuse. The drug has a currently accepted medical use in treatment in the United States or a currently accepted medical use with severe restrictions. Abuse of the drug may lead to severe psychological or physical dependence.
Hydromorphone hydrochloride
- Imprint(s):
- M
4
|
- Strength(s):
- 4 mg
|
- Color:
- White
|
- Size:
- 7.00 mm
|
- Shape:
- Round
|
- Availability:
- Prescription only
|
- Inactive Ingredients:Generic Naprosyn (Naproxen)
-
- lactose monohydrate
-
- magnesium stearate
- microcrystalline cellulose
- stearic acid
- Drug Class:
- Narcotic analgesics
- Pregnancy Category:
- C – Risk cannot be ruled out
- CSA Schedule:
- 2 – High potential for abuse
- Manufacturer:
- Mallinckrodt Pharmaceuticals
- National Drug Code (NDC):
- 00406-3244
- Other Manufacturers / Repackagers:
Posted by glbgn754
on January 31, 2012
Uncategorized /
No Comments
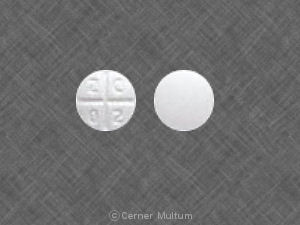
Pill imprint Z C 0 2 has been identified as Promethazine hydrochloride 25 mg.
Promethazine is used in the treatment of nausea/vomiting; vertigo; sedation; motion sickness; opiate adjunct (and more), and belongs to the drug classes antihistamines, phenothiazine antiemetics. Risk cannot be ruled out during pregnancy.
Promethazine 25 mg is not subject to the Controlled Substances Act.
Promethazine hydrochloride
- Imprint(s):
- Z C 0 2
|
- Strength(s):
- 25 mg
|
- Color:
- White
|
- Size:
- N/A
|
- Shape:
- Round
|
- Availability:
- Prescription only
|
- Antihistamines
-
Phenothiazine antiemetics
- Pregnancy Category:
- C – Risk cannot be ruled out
- CSA Schedule:
- N – Not a controlled drug
- Manufacturer:
- Zydus Pharmaceuticals
- National Drug Code (NDC):
- 93 896
- Other Manufacturers / Repackagers:M 745
Posted by glbgn754
on January 31, 2012
Uncategorized /
No Comments
Pill imprint GG 296 has been identified as Loratadine 10 mg.
Loratadine is used in the treatment of hay fever; urticaria and belongs to the drug class antihistamines. There is no proven risk in humans during pregnancy.
Loratadine 10 mg is not subject to the Controlled Substances Act.
- Imprint(s):
- GG 296
|
- Strength(s):
- 10 mg
- G 164
|
- Color:
- White
|
- Size:
- 6.00 mm
|
- Shape:
- Round
|
- Availability:
- Rx and/or OTC
|
- Inactive Ingredients:
-
- lactose monohydrate
- magnesium stearate
- microcrystalline cellulose
- sodium starch glycolate type A potato
- Drug Class:
- Antihistamines
- Pregnancy Category:
- B – No proven risk in humans
- CSA Schedule:
- N – Not a controlled drug
- Manufacturer:
- Sandoz Pharmaceuticals Inc.
- National Drug Code (NDC):
- 00781-5077
- Other Manufacturers / Repackagers:
Posted by glbgn754
on January 31, 2012
Uncategorized /
No Comments
Pill imprint M 2 has been identified as Hydromorphone hydrochloride 2 mg.
Hydromorphone is used in the treatment of pain; cough; anesthetic adjunct and belongs to the drug class narcotic analgesics. Risk cannot be ruled out during pregnancy.LOGO TH
Hydromorphone 2 mg has a high potential for abuse. The drug has a currently accepted medical use in treatment in the United States or a currently accepted medical use with severe restrictions. Abuse of the drug may lead to severe psychological or physical dependence.

- Imprint(s):
- M
2
|
- Strength(s):
- 2 mg
|
- Color:
- White
|
- Size:
- 6.00 mm
|
- Shape:
- Round
|
- Availability:
- Prescription only
|
Posted by glbgn754
on January 31, 2012
Uncategorized /
No Comments
Pill imprint GG 225 has been identified as Promethazine hydrochloride 25 mg.
Promethazine is used in the treatment of nausea/vomiting; vertigo; sedation; motion sickness; opiate adjunct (and more), and belongs to the drug classes antihistamines, phenothiazine antiemetics. Risk cannot be ruled out during pregnancy.M 424
Promethazine 25 mg is not subject to the Controlled Substances Act.

Promethazine hydrochloride
- Imprint(s):
- GG 225
|
- Strength(s):
- 25 mg
|
- Color:
- White
|
- Size:
- N/A
|
- Shape:
- Round
|
- Availability:
- Prescription only
|
- Other Manufacturers / Repackagers:
-
| NDC Code |
Manufacturer / Repackager |
| 63739-0213 |
McKesson Packaging Services |
| 54569-1754 |
A S Medication Solutions LLC (repackager) |
| 54569-4168 |
A S Medication Solutions LLC (repackager) |
| 55289-0464 |
PDRX Pharmaceuticals Inc (repackager) |
| 68115-0302 |
Dispensexpress Inc (repackager) |
| 66267-0177 |
Nucare Pharmaceuticals Inc (repackager) |
| 57866-4379 |
Direct Dispensing Inc (repackager) |
| 49999-0090 |
Lake Erie Medical and Surgical Supply (repackager) |
| Note: Inactive ingredients may vary. |
Posted by glbgn754
on January 31, 2012
Uncategorized /
No Comments
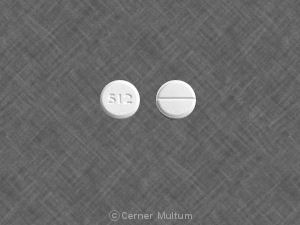
Pill imprint 512 has been identified as Acetaminophen and oxycodone hydrochloride 325 mg / 5 mg.
Acetaminophen/oxycodone is used in the treatment of pain and belongs to the drug class narcotic analgesic combinations. Risk cannot be ruled out during pregnancy.
Acetaminophen/oxycodone 325 mg / 5 mg has a high potential for abuse. The drug has a currently accepted medical use in treatment in the United States or a currently accepted medical use with severe restrictions. Abuse of the drug may lead to severe psychological or physical dependence.
Acetaminophen and oxycodone hydrochloride
- Imprint(s):
- 512
|
- Strength(s):
- 325 mg / 5 mg
|
- Color:
- White
|
- Size:
- 12.00 mm
|
- Shape:
- Round
|
- Availability:
- Prescription only
|
- Inactive Ingredients:
-
- crospovidone
- microcrystalline cellulose
- povidone
- corn starch
- silicon dioxide
- stearic acid
- Drug Class:


APO CI 10
Pill imprint APO CI 10 has been identified as Citalopram hydrobromide 10 mg.
Citalopram is used in the treatment of depression; anxiety and stress; postpartum depression; severe mood dysregulation; obsessive compulsive disorder (and more), and belongs to the drug class selective serotonin reuptake inhibitors. Risk cannot be ruled out during pregnancy.
Citalopram 10 mg is not subject to the Controlled Substances Act.
See also related documents.
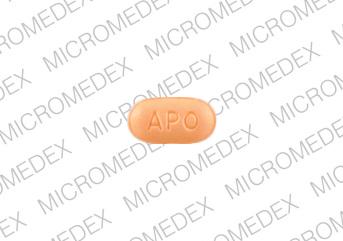

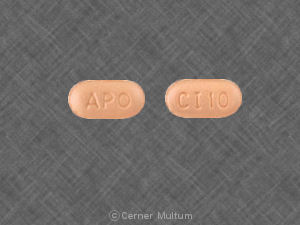
Citalopram hydrobromide
- Imprint(s):
- APO
CI 10
|
- Strength(s):
- 10 mg
|
- Color:
- Beige
|
- Size:
- 7.00 mm
|
- Shape:
- Elliptical / Oval
|
- Availability:
- Prescription only
|
- Inactive Ingredients:
-
- croscarmellose sodium
- lactose monohydrate
- magnesium stearate
- microcrystalline cellulose
- hydroxyethyl cellulose
- polyethylene glycol
- ferric oxide red
- titanium dioxide
- ferric oxide yellow
- Drug Class:
- Selective serotonin reuptake inhibitors
- Pregnancy Category:
- C – Risk cannot be ruled out
- CSA Schedule:
- N – Not a controlled drug
- Manufacturer:
- Apotex Corp.
- National Drug Code (NDC):
- 60505-2518
- Narcotic analgesic combinations
- Pregnancy Category:
- C – Risk cannot be ruled out
- CSA Schedule:
- 2 – High potential for abuse
- Manufacturer:
- Mallinckrodt Pharmaceuticals
- National Drug Code (NDC):
- 00406-0512
View Drug DetailsPrint Page