Generic Name: acetaminophen/oxycodone
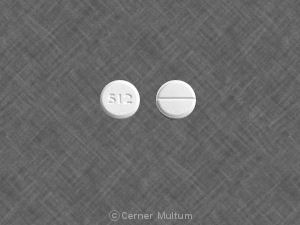
Pill imprint Endo 602 has been identified as Endocet 325 mg / 5 mg.
Endocet is used in the treatment of pain and belongs to the drug class narcotic analgesic combinations. Risk cannot be ruled out during pregnancy.
Endocet 325 mg / 5 mg has a high potential for abuse. The drug has a currently accepted medical use in treatment in the United States or a currently accepted medical use with severe restrictions. Abuse of the drug may lead to severe psychological or physical dependence.
- Generic Name:
- acetaminophen/oxycodone
|
|
|
|
|
|
- Inactive Ingredients:
-
- silicon dioxide
- croscarmellose sodium
- crospovidone
- microcrystalline cellulose
- povidone
- corn starch
- stearic acid
- Drug Class:
- Narcotic analgesic combinations
- Pregnancy Category:
- C – Risk cannot be ruled out
- CSA Schedule:
- 2 – High potential for abuse
- Manufacturer:
- Endo Pharmaceuticals Inc.