Posted by glbgn754
on November 01, 2013
Uncategorized /
Comments Off on National Drug Code Directory
The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of all drugs manufactured, prepared, propagated, compounded, or processed by it for commercial distribution. (See Section 510 of the Federal Food, Drug, and Cosmetic Act (Act) (21 U.S.C. § 360)). Drug products are identified and reported using a unique, three-segment number, called the National Drug Code (NDC), which serves as a universal product identifier for drugs. FDA publishes the listed NDC numbers and the information submitted as part of the listing information in the NDC Directory which is updated daily.
The information submitted as part of the listing process, the NDC number, and the NDC Directory are used in the implementation and enforcement of the Act.
(NOTE: New field “ProductID” added 8/23/2013 in Product and package files.)
–
New NDC Directory
Search National Drug Code Directory
Searchable database
NDC Database File (Zip Format) (ZIP – 13.6MB) Updated 10/31/2013
NDC Product File Definitions
Product File Data Elements, Definitions, and Notes
NDC Package File Definitions
Package File Data Elements, Definitions, and Notes
–
–
Old NDC Directory
Old NDC Database File (Zip Format) (ZIP – 4.8MB) [ARCHIVED]
Data Files Updated through July 2012 (Final Update)
Search OLD National Drug Code Directory
–
Current regulations require a registered establishment to update its drug listing data in June and December of each year, or, at the discretion of the establishment, when a change occurs (see 21 C.F.R. § 207.30); therefore, FDA may not yet have been notified of recent changes before updating the NDC Directory. FDA makes every effort to prevent errors and discrepancies in the NDC Directory data. Users who detect any errors are requested to contact:
Food and Drug Administration
Center for Drug Evaluation and Research
Office of Compliance, Immediate Office
Drug Registration and Listing Team
10903 New Hampshire Ave
Silver Spring, MD 20993-0002
Email: eDRLS@fda.hhs.gov
Posted by glbgn754
on November 01, 2013
Uncategorized /
Comments Off on 30 M (Morphine sulfate SR 30 mg)
Morphine is used in the treatment of chronic pain; pain and belongs to the drug class narcotic analgesics. Risk cannot be ruled out during pregnancy. Morphine 30 mg has a high potential for abuse. The drug has a currently accepted medical use in treatment in the United States or a currently accepted medical use with severe restrictions. Abuse of the drug may lead to severe psychological or physical dependence.
See also related documents.
Ads by Google
Визитки – Сделай Онлайн
Сделай Визитки сам в конструкторе. Напечатаем за 1 день! От 399 руб.
PrintClick.Ru/Визитки_Онлайн
Images for 30 M (Morphine sulfate SR 30 mg)
Morphine sulfate SR 30 mg 30 M
Morphine sulfate SR 30 mg 30 M Front
Morphine sulfate SR 30 mg 30 M Back
Morphine sulfate SR
Imprint:
30
M
Strength:
30 mg
Color:
Purple
Size:
7.00 mm
Shape:
Round
Availability:
Prescription only
Drug Class:
Narcotic analgesics
Pregnancy Category:
C – Risk cannot be ruled out
CSA Schedule:
2 – High potential for abuse
Manufacturer:
Mallinckrodt Pharmaceuticals
National Drug Code (NDC):
00406-8330
Inactive Ingredients:
hypromellose 2208 (15000 mPa.s)
lactose monohydrate
magnesium stearate
polyethylene glycol
polydextrose[google-map-v3 shortcodeid=”TO_BE_GENERATED” width=”350″ height=”350″ zoom=”12″ maptype=”roadmap” mapalign=”center” directionhint=”false” language=”default” poweredby=”false” maptypecontrol=”true” pancontrol=”true” zoomcontrol=”true” scalecontrol=”true” streetviewcontrol=”true” scrollwheelcontrol=”false” draggable=”true” tiltfourtyfive=”false” enablegeolocationmarker=”false” enablemarkerclustering=”false” addmarkermashup=”false” addmarkermashupbubble=”false” addmarkerlist=”Morphine sulfate SR 30 mg){}beachvolleyball.png” bubbleautopan=”true” distanceunits=”miles” showbike=”false” showtraffic=”false” showpanoramio=”false”]
silicon dioxide
titanium dioxide
triacetin
FD&C Blue No. 2
D&C Red No. 1
Posted by glbgn754
on November 01, 2013
Uncategorized /
Comments Off on IP 190 500 (Naproxen 500 mg)
Naproxen is used in the treatment of back pain; osteoarthritis; pain; ankylosing spondylitis; gout, acute (and more), and belongs to the drug class nonsteroidal anti-inflammatory agents. Risk cannot be ruled out during pregnancy. Naproxen 500 mg is not subject to the Controlled Substances Act.
See also related documents
Ads by Google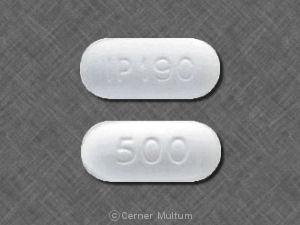
Телевизор в Эльдорадо
Большой выбор телевизоров по отличным ценам. Покупай со скидкой!
www.eldorado.ru/Телевизор
Images for IP 190 500 (Naproxen 500 mg)
Naproxen 500 mg IP 190 500
Naproxen 500 mg IP 190 500
Naproxen 500 mg IP 190 500
Naproxen 500 mg IP 190 500 Front
Naproxen 500 mg IP 190 500 Back
Naproxen
Imprint:
IP 190
500
Strength:
500 mg
Color:
White
Shape:
Elliptical / Oval
Availability:
Rx and/or OTC
Drug Class:
Nonsteroidal anti-inflammatory agents
Pregnancy Category:
C – Risk cannot be ruled out
CSA Schedule:
N – Not a controlled drug
Manufacturer:
Akyma Pharmaceuticals


