Posted by glbgn754
on July 31, 2013
Uncategorized /
Comments Off on PROCARDIA XL 30 (Procardia XL 30 mg)
Pill imprint PROCARDIA XL 30 has been identified as Procardia XL 30 mg.
Procardia XL is used in the treatment of high blood pressure and belongs to the drug class calcium channel blocking agents. Risk cannot be ruled out during pregnancy. Procardia XL 30 mg is not subject to the Controlled Substances Act.
See also related documents.
Ads by Google
Mandela Day
Learn more about great ideas that change the world for the better!
siemens.com/answers/mandela-school
Images for PROCARDIA XL 30 (Procardia XL 30 mg)
Procardia XL 30 mg PROCARDIA XL 30 Front
Procardia XL 30 mg PROCARDIA XL 30 Back
Procardia XL 30 mg PROCARDIA XL 30
Procardia XL
Generic Name:
nifedipine
Imprint:
PROCARDIA XL 30
Strength:
30 mg
Color:
Pink
Size:
9.00 mm
Shape:
Round
Availability:
Prescription only
Drug Class:
Calcium channel blocking agents
Pregnancy Category:
C – Risk cannot be ruled out
CSA Schedule:
N – Not a controlled drug
Manufacturer:
Pfizer U.S. Pharmaceuticals Group
National Drug Code (NDC):
00069-2650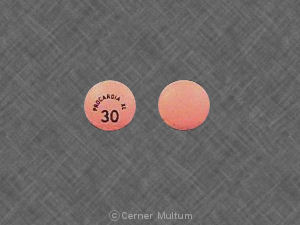
Inactive Ingredients:
cellulose acetate
hydroxypropyl cellulose
hypromelloses
magnesium stearate
polyethylene glycol
ferric oxide red
sodium chloride
titanium dioxide
Other Manufacturers / Repackagers:
NDC Code Manufacturer / Repackager
54868-1006 Physicians Total Care Inc (repackager)
55289-0323 PDRX Pharmaceuticals Inc (repackager)
00247-0451 Prescript Pharmaceuticals (repackager)
55289-0798 PDRX Pharmaceuticals Inc (repackager)
Posted by glbgn754
on July 31, 2013
Uncategorized /
Comments Off on PX 3 (Pramipexole dihydrochloride 1 mg)
Pill imprint PX 3 has been identified as Pramipexole dihydrochloride 1 mg.
Pramipexole is used in the treatment of restless legs syndrome; periodic limb movement disorder; parkinson’s disease; tardive dyskinesia and belongs to the drug class dopaminergic antiparkinsonism agents. Risk cannot be ruled out during pregnancy. Pramipexole 1 mg is not subject to the Controlled Substances Act.
See also related documents.
Ads by Google
Кредит до 1500000 рублей
Кредит без поручителей? Возможно. Заполните заявку прямо Сейчас!
vbanki.ru
Images for PX 3 (Pramipexole dihydrochloride 1 mg)
Pramipexole dihydrochloride 1 mg PX 3
Pramipexole dihydrochloride 1 mg PX 3
Pramipexole dihydrochloride
Imprint:
PX 3
Strength:
1 mg
Color:
White
Shape:
Elliptical / Oval
Availability:
Prescription only
Drug Class:
Dopaminergic antiparkinsonism agents
Pregnancy Category:
C – Risk cannot be ruled out
CSA Schedule:
N – Not a controlled drug
Manufacturer:
Glenmark Generics Inc.
National Drug Code (NDC):
68462-0333
Posted by glbgn754
on July 31, 2013
Uncategorized /
Comments Off on PRASCO 327 (Phenylephrine and guaifenesin SR 30 mg / 900 mg)
Pill imprint PRASCO 327 has been identified as Phenylephrine and guaifenesin SR 30 mg / 900 mg.
Guaifenesin/phenylephrine is used in the treatment of cough and nasal congestion; sinus symptoms and belongs to the drug class upper respiratory combinations. Risk cannot be ruled out during pregnancy. Guaifenesin/phenylephrine 30 mg / 900 mg is not subject to the Controlled Substances Act.
See also related documents.
Ads by Google
Letter to Semy
Access to secondary education now, for a sustainable future in Africa!
siemens.com/answers/mandela-school
Images for PRASCO 327 (Phenylephrine and guaifenesin SR 30 mg / 900 mg)
Phenylephrine and guaifenesin SR 30 mg / 900 mg PRASCO 327
Phenylephrine and guaifenesin SR
PRASCO
327
Strength:
30 mg / 900 mg
Color:
White
Shape:
Elliptical / Oval
Availability:
Rx and/or OTC
Drug Class:
Upper respiratory combinations
Pregnancy Category:
C – Risk cannot be ruled out
CSA Schedule:
N – Not a controlled drug
Manufacturer:
Prasco Laboratories
Posted by glbgn754
on July 31, 2013
Uncategorized /
Comments Off on Pfizer PGN 200 (Lyrica 200 mg)
Pill imprint Pfizer PGN 200 has been identified as Lyrica 200 mg.
Lyrica is used in the treatment of neuropathic pain; fibromyalgia; diabetic peripheral neuropathy; postherpetic neuralgia; neuralgia (and more), and belongs to the drug class gamma-aminobutyric acid analogs. Risk cannot be ruled out during pregnancy. Lyrica 200 mg has a low potential for abuse relative to the drugs in schedule 4. The drug has a currently accepted medical use in treatment in the United States. Abuse of the drug may lead to limited physical dependence or psychological dependence relative to the drugs in schedule 4.
See also related documents.
Ads by Google
Letter to Semy
Access to secondary education now, for a sustainable future in Africa!
siemens.com/answers/mandela-school
Images for Pfizer PGN 200 (Lyrica 200 mg)
Lyrica 200 mg Pfizer PGN 200
Lyrica 200 mg Pfizer PGN 200
Lyrica
Generic Name:
pregabalin
Imprint:
Pfizer
PGN 200
Strength:
200 mg
Color:
Orange
Size:
19.00 mm
Shape:
Capsule-shape
Availability:
Prescription only
Drug Class:
Gamma-aminobutyric acid analogs
Pregnancy Category:
C – Risk cannot be ruled out
CSA Schedule:
5 – Some potential for abuse
Manufacturer:
Pfizer U.S. Pharmaceuticals Group
National Drug Code (NDC):
00071-1017
Posted by glbgn754
on July 31, 2013
Uncategorized /
Comments Off on PFIZER 073 TERRAMYCIN (Terramycin 250 MG)
Pill imprint PFIZER 073 TERRAMYCIN has been identified as Terramycin 250 MG.
and belongs to the drug class tetracyclines. There is positive evidence of human fetal risk during pregnancy. Terramycin 250 MG is not subject to the Controlled Substances Act.
See also related documents.
Ads by Google
Letter to Semy
Access to secondary education now, for a sustainable future in Africa!
siemens.com/answers/mandela-school
Images for PFIZER 073 TERRAMYCIN (Terramycin 250 MG)
Terramycin 250 MG PFIZER 073 TERRAMYCIN
Terramycin
Generic Name:
oxytetracycline
Imprint:
PFIZER 073
TERRAMYCIN
Strength:
250 MG
Color:
Yellow
Shape:
Capsule-shape
Availability:
Prescription only
Drug Class:
Tetracyclines
Pregnancy Category:
D – Positive evidence of risk
CSA Schedule:
N – Not a controlled drug
Posted by glbgn754
on July 31, 2013
Uncategorized /
Comments Off on PFIZER 095 VIBRA (Vibramycin hyclate 100 MG)
Pill imprint PFIZER 095 VIBRA has been identified as Vibramycin hyclate 100 MG.
Vibramycin is used in the treatment of bacterial infection; acne; upper respiratory tract infection; urinary tract infection; skin infection (and more), and belongs to the drug classes miscellaneous antimalarials, tetracyclines. There is positive evidence of human fetal risk during pregnancy. Vibramycin 100 MG is not subject to the Controlled Substances Act.
See also related documents.
Ads by Google
Mandela Day
Learn more about great ideas that change the world for the better!
siemens.com/answers/mandela-school
Images for PFIZER 095 VIBRA (Vibramycin hyclate 100 MG)
Vibramycin hyclate 100 MG PFIZER 095 VIBRA
Vibramycin hyclate
Generic Name:
doxycycline
Imprint:
PFIZER 095
VIBRA
Strength:
100 MG
Color:
Blue
Shape:
Capsule-shape
Availability:
Prescription only
Drug Class:
Miscellaneous antimalarials
Tetracyclines
Pregnancy Category:
D – Positive evidence of risk
CSA Schedule:
N – Not a controlled drug
Manufacturer:
Pfizer U.S. Pharmaceuticals Group
National Drug Code (NDC):
00069-0950
Inactive Ingredients:
FD&C Blue No. 1
magnesium stearate
microcrystalline cellulose
sodium lauryl sulfate
Posted by glbgn754
on July 31, 2013
Uncategorized /
Comments Off on PFIZER 099 (Vibra-tabs 100 mg)
Pill imprint PFIZER 099 has been identified as Vibra-tabs 100 mg.
Vibra-Tabs is used in the treatment of bacterial infection; acne; upper respiratory tract infection; urinary tract infection; skin infection (and more), and belongs to the drug classes miscellaneous antimalarials, tetracyclines. There is positive evidence of human fetal risk during pregnancy. Vibra-Tabs 100 mg is not subject to the Controlled Substances Act.
Posted by glbgn754
on July 31, 2013
Uncategorized /
Comments Off on PFIZER 376 (Renese 2 mg)
Pill imprint PFIZER 376 has been identified as Renese 2 mg.
Renese is used in the treatment of high blood pressure; edema and belongs to the drug class thiazide diuretics. There is positive evidence of human fetal risk during pregnancy. Renese 2 mg is not subject to the Controlled Substances Act.
See also related documents.
Ads by Google
Mandela Day
Learn more about great ideas that change the world for the better!
siemens.com/answers/mandela-school
Images for PFIZER 376 (Renese 2 mg)
Renese 2 mg PFIZER 376
Renese
Generic Name:
polythiazide
Imprint:
PFIZER 376
Strength:
2 mg
Color:
Yellow
Shape:
Round
Availability:
Prescription only
Drug Class:
Thiazide diuretics
Pregnancy Category:
D – Positive evidence of risk
CSA Schedule:
N – Not a controlled drug
Manufacturer:
Pfizer U.S. Pharmaceuticals Group
National Drug Code (NDC):
00069-3760
Inactive Ingredients:
dibasic calcium phosphate
lactose
magnesium stearate
polyethylene glycol
sodium lauryl sulfate
starch
vanillin
FD&C Yellow No. 6
FD&C Yellow No. 10
Posted by glbgn754
on July 31, 2013
Uncategorized /
Comments Off on Pfizer CDT 108 (Caduet 10 mg / 80 mg)
Pill imprint Pfizer CDT 108 has been identified as Caduet 10 mg / 80 mg.
Caduet is used in the treatment of high blood pressure; angina and belongs to the drug classes antihyperlipidemic combinations, antihypertensive combinations. Not for use in pregnancy. Caduet 10 mg / 80 mg is not subject to the Controlled Substances Act.
See also related documents.
Ads by Google
Mandela Day
Learn more about great ideas that change the world for the better!
siemens.com/answers/mandela-school
Images for Pfizer CDT 108 (Caduet 10 mg / 80 mg)
Caduet 10 mg / 80 mg Pfizer CDT 108
Caduet 10 mg / 80 mg Pfizer CDT 108
Caduet 10 mg / 80 mg Pfizer CDT 108 Front
Caduet 10 mg / 80 mg Pfizer CDT 108 Back
Caduet
Generic Name:
amlodipine/atorvastatin
Imprint:
Pfizer
CDT 108
Strength:
10 mg / 80 mg
Color:
Blue
Size:
17.00 mm
Shape:
Elliptical / Oval
Availability:
Prescription only
Drug Class:
Antihyperlipidemic combinations
Antihypertensive combinations
Pregnancy Category:
X – Not for use in pregnancy
CSA Schedule:
N – Not a controlled drug
Manufacturer:
Pfizer U.S. Pharmaceuticals Group
National Drug Code (NDC):
00069-2270
Inactive Ingredients:
calcium carbonate
croscarmellose sodium
microcrystalline cellulose
polysorbate 80
hydroxypropyl cellulose
water
silicon dioxide
magnesium stearate
polyvinyl alcohol
titanium dioxide
polyethylene glycol 3000
magnesium silicate
FD&C Blue No. 2
Posted by glbgn754
on July 31, 2013
Uncategorized /
Comments Off on Pfizer CHX 0.5 (Chantix 0.5 mg)
Pill imprint Pfizer CHX 0.5 has been identified as Chantix 0.5 mg.
Chantix is used in the treatment of smoking cessation and belongs to the drug class smoking cessation agents. Risk cannot be ruled out during pregnancy. Chantix 0.5 mg is not subject to the Controlled Substances Act.
See also related documents.
Ads by Google
/answers Magazine
South African youth education helps sustainable development in Africa!
siemens.com/answers/mandela-school
Images for Pfizer CHX 0.5 (Chantix 0.5 mg)
Chantix 0.5 mg Pfizer CHX 0.5 Front
Chantix 0.5 mg Pfizer CHX 0.5 Back
Chantix 0.5 mg Pfizer CHX 0.5
Chantix
Generic Name:
varenicline
Imprint:
Pfizer
CHX 0.5
Strength:
0.5 mg
Color:
White
Size:
8.00 mm
Shape:
Elliptical / Oval
Availability:
Prescription only
Drug Class:
Smoking cessation agents
Pregnancy Category:
C – Risk cannot be ruled out
CSA Schedule:
N – Not a controlled drug
Manufacturer:
Pfizer U.S. Pharmaceuticals Group
National Drug Code (NDC):
00069-0468
Inactive Ingredients:
microcrystalline cellulose
calcium phosphate dibasic anhydrous
croscarmellose sodium
silicon dioxide
magnesium stearate





